Plastic: сheap, convenient, hazardous
Plastic is convenient and cheap. It also decomposes slowly—so it will stay with us for quite a long time. So just how ubiquitous is it?

The extent of the problem with plastic
Can you try to guess how much plastic we make for an average person?
Turns out it is 68 kg for every American citizen each year. When talking about Europeans, the figure is about 50 kg of various plastics per person per year. It can get lower than that in other countries, but overall we produce up to 380 million tons of plastic each year globally.
Looks like plastic is a pretty popular material, isn’t it? Why is it so popular? This is a question we’ll try to answer here.
Plastic is pretty flexible and moldable. It can be shaped into almost anything, from a pipe to a cup. Microscopic plastic particles are sometimes added to products that may not seem obvious to have plastics included—even cosmetics and hygiene products.

Plastic is pretty durable, and also cheap. In fact, it is one of the most cost-effective materials for storing and packaging.
The exact numbers are not that easy to obtain, but here are some statistics:
- Nearly 500 billion plastic bottles were sold in 2016, equalling 1 million per minute or 20 000 per second.
- Up to 5 trillion plastic bags are used yearly. Notice that it’s not millions or billions, but trillions.
- Nearly 500 billion disposable cups are thrown away every year.
The production only increases, so these numbers may get even larger with every passing year.
The nature of plastics
Plastic is mainly made of polymers. Polymers are long molecules that consist of repeated identical elements (each element is called a monomer).

The picture represents the formula of Polytetrafluoroethylene, more commonly known as Teflon (many commercial plastics have a long scientific name and a short commercial name that is usually a trademark). Teflon is a very popular plastic used to create a non-sticking surface for frying pans and other cookware. Teflon was discovered by accident. Roy J. Plunkett, a researcher at DuPont in the 1930s, was looking for a non-toxic refrigeration agent. One of his experiments yielded a white powder that didn’t do well as a refrigerant. But he checked the powder for other properties anyway—and discovered it to be resistant to heat and chemically inert (that is, not eager to interact with other chemicals). The substance also had very low surface friction, meaning that other things would not stick to it. Sensing a great potential, Plunkett made a polymer out of it.
The formula of the monomer determines the type of plastic. The types vary by composition and purpose. Here is some of the most common:
- Polycarbonate is used in sunglasses, DVDs, cars, planes, smartphones, etc.
- Polyethylene is used for shopping bags and bottles.
- Polyvinyl chloride is used for shower curtains and pipes.
- Polyester is used in clothing and other textiles
- Polystyrene is used for disposable dishes—including the ever-popular coffee cups.
- Polyamide is a versatile material used particularly everywhere, from gyms to kitchens.
You can identify these types by a code, which is usually marked on a product.
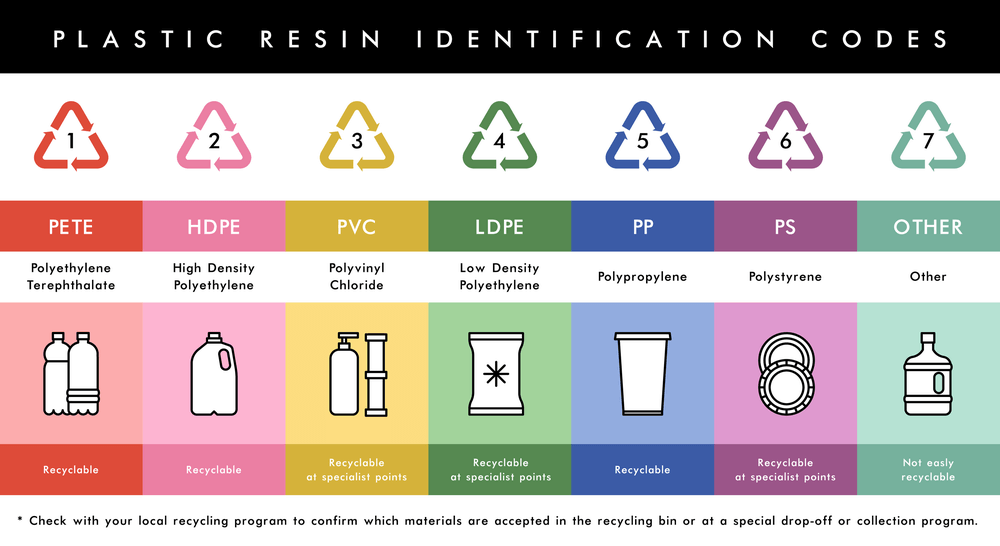
There are dozens more types of plastics, all of them with different properties.
For example, thermoplastics can be heated to change their shape. They’re widely used for marking roads and crossings.

Conductive polymers are plastics that conduct electricity. They are used for electroluminescent lamps, solar panels, and biosensors.

Polyethylene has many uses. As we already know, thin plastic bags and stretch wrap are made of its linear low-density kind. High-density polyethylene is handy for many engineering solutions, like flexible pipes or crates. It can even be useful for making chairs.

As you can see, plastics can be modified to suit different purposes. It is not a single material—there are many kinds of plastics with different properties.
The strength and plasticity of a polymer molecule make plastic a handy solution for a vast variety of needs. This is why you can see it utilized almost everywhere. Flying on a plane or driving a car, plastic is everywhere around you.

The making of plastic
As you already know, the structure of plastic is based on long molecules called polymers. Polymers are made of many same blocks, called monomers. So, there are several stages of making any plastic:
- Take something as a source: fossil fuels and plant components are the most common sources. Now process the source to extract fractions out of their hydrocarbon chains. From this process, you’ll get some quantity of a monomer: like ethylene or propylene.
- Use a catalyst on these monomers to kickstart a chemical process known as polymerization. Voila! Now you have a polymer, like polyethylene or polypropylene.

It usually comes in granules or pellets, because that way it is easier to transport to manufacturers.
The manufacturers can melt it together, dye it, shape it, or add some other chemicals to modify its properties. For example, you almost always need something to make the structure more stable.
The chemicals you add at this stage can also be harmful to human health and the environment. Make a mental note of this—because we’ll get back to it a bit later.
Who was the first to come up with the process? Alexander Parkes in 1855 made the first semi-organic plastic. He used cellulose, a component of plant cells, as the source. Course block
We now know this material as celluloid—it is used, for example, to make tennis balls.

The plastic we use today is mostly fully synthetic—that is, made of non-organic components. The first to make synthetic plastic was Leo Baekeland in the early 1900s. The scientific name of plastic corresponds to its molecular formula and structure. The material synthesized by Baekeland is named polyoxybenzylmethylenglycolanhydride (kudos to you if you were able to read it all in one go). As you can guess, its formula and structure are pretty complex. This plastic, remarkable for its heat resistance and nonconductivity, was used throughout the first half of the 20th century under the name Bakelite.
Baekeland, who is often called the father of the plastic industry, used phenol—a byproduct of burning oil or coal. His material was cheap and versatile, as is often the case with synthetic plastics nowadays.
In 1959 the world produced 2 million tons of plastic. By 2018, this figure went up to 380 million tons. This number is expected to double by 2050.
Surely, all of this plastic has to go somewhere when we no longer need it. And this is the root of the problem.